Interstitial Sites or Interstitial Voids:
In the close packing of spheres, certain hollows are left vacant. These holes or voids in the crystals are called interstitial sites or interstitial voids. Two important interstitial sites are
(1) Tetrahedral
(2) Octahedral.
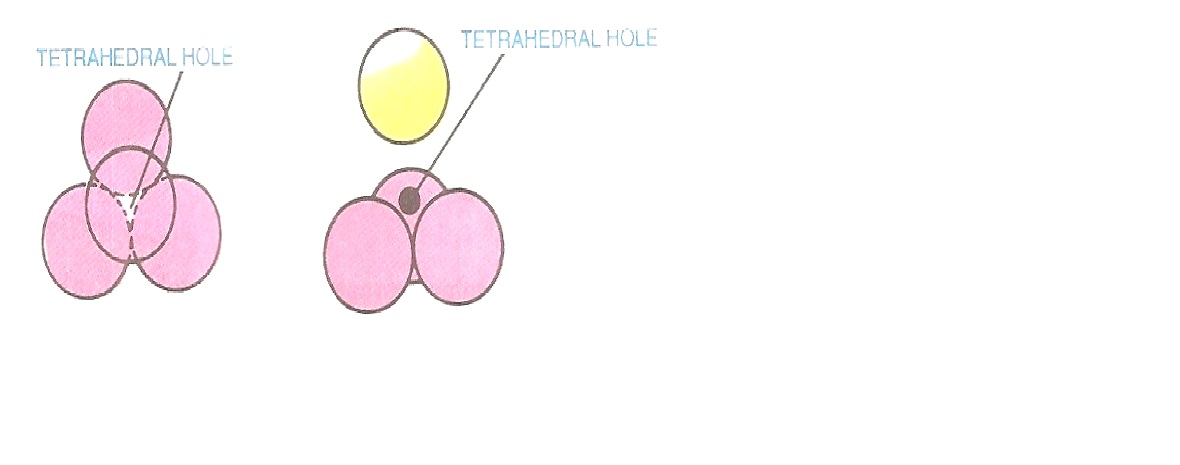
Tetrahedral site:
A sphere in the second layer is placed above three spheres touching one another in the first layer. This is shown in Fig. 2.7. The centres of these spheres lie at the apices of a tetrahedron. It may be noted that the shape of the void is not tetrahedral, but the arrangement around this void is tetrahedral. Thus, the vacant space among four spheres having tetrahedral arrangement is called tetrahedral site or tetrahedral hole.
Octahedral site:
This type of Void is formed at the centre of six spheres. It is shown in Fig. 2.8. From the figure, it is clear that each octahedral site is produced by two sets of equilateral triangles which point in opposite directions. Thus, the void farmed by two equilateral triangles with apices in opposite direction is called octahedral site or octahedral hole. This site is, therefore, surrounded by 6 spheres lying at the vertices of a regular octahedron.
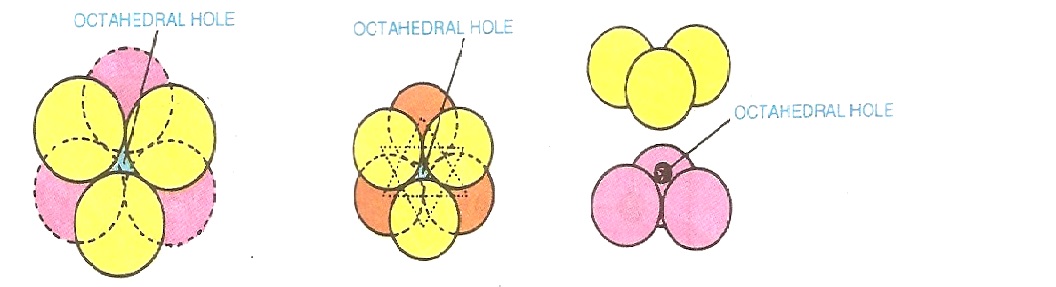
Fig: 2.8. Octahedral site.
Number of octahedral and tetrahedral sites:
There are two tetrahedral sites for each sphere and there is only one octahedral site for each sphere. Thus, in a close packed structure of N spheres, there are 2N tetrahedral sites and N octahedral sites.
Coordination Number:
The number of spheres which are touching a given sphere is called the coordination number. In hcp and ccp arrangements, a sphere is in direct contact with 6 other spheres in its own layer. It touches three spheres in the layer above it and three spheres in the layer below it. Thus, its coordination number in hcp and ccp arrangements is 12 as shown in Fig. 2.9.
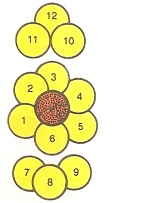
Fig: 2.9 coordination number of 12 in hcp & ccp.