Alkenes:
Alkenes are hydrocarbons that contain carbon-carbon double bonds in their molecules. They are also called Olefines as lower members of alkenes form oily products on treatment with chlorine or bromine. Alkenes contain two hydrogen atoms less than alkanes and thus, called unsaturated hydrocarbons. Alkenes can be represented by a general formula,
Cn H2n
Where, n = 2, 3, 4… etc.
Ethylene is the first member of the series and propylene is the second member of the series. These are represented as:
CH2 = CH2 CH3—CH = CH2
Ethylene Propylene
Nomenclature of alkene:
There are two ways of naming alkenes:
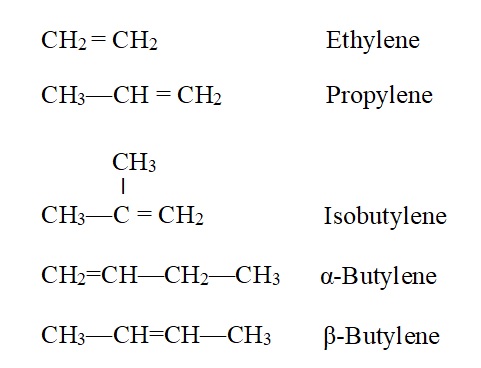
i) Common system:
The common names of the first four members are derived from those of the corresponding alkanes by changing the ending –ane to –ylene. Greek letters are used to distinguish isomers having double bond at the first (α) or the second (β) carbon of the chain. For example,
 ii) IUPAC- System:
ii) IUPAC- System:
The IUPAC names of alkenes are derived from the corresponding alkanes by changing the ending –ane to –ene. The IUPAC names of higher alkenes are obtained by applying the following rules:
1) The name of the hydrocarbon is based on the parent alkene having the longest carbon chain of which double bond is a part.
2) This chain is numbered from the end near the double bond and its position is indicated by the number of the carbon atom at which the double bond originates.
3) The name of the parent alkene with the position number of the double bond is written first and then the names of other substituents prefixed to it. e.g.
![]()
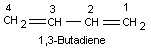
4) When there are two or three double bonds in a molecule, the ending –ane of the corresponding alkane is replaced by –adiene or –atriene to get the name of the hydrocarbon.
 Structure of double bond (ethylene):
Structure of double bond (ethylene):
Let us consider (H2C=CH2) for illustrating the orbital make up of alkenes.
In ethylene carbon atoms are sp2 hybridized and attached to each other through a σ bond and a π bond. The σ bond results from the overlap of two sp2 hybrid orbitals (i.e. one from each carbon). The π bond is formed from overlap of the unhybridized p-orbitals.
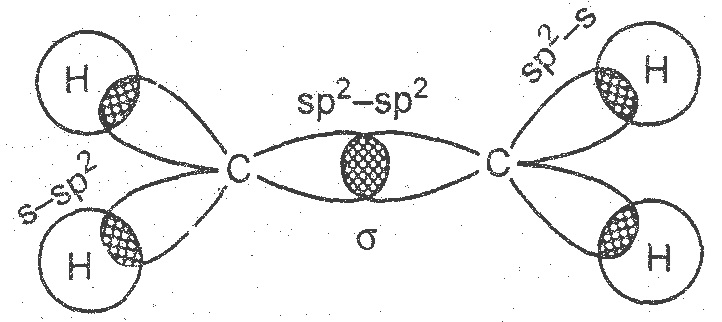
The remaining sp2 orbitals form σ bonds with either carbon or hydrogen atoms. Ethylene is a planar molecule.
Note:
i) The carbon-carbon double bond in alkenes is consist of one σ bond and one π bond.
ii) Alkenes are more reactive than alkanes. This is due to the availability of the more exposed π electrons.