Aromatic Hydrocarbons:
Benzene has a cyclic, planar, hexagonal structure. All carbon-carbon bond lengths are identical and are intermediate between C—C single bonds and C=C double bonds. Benzene does not have alternate single and double bonds. All hydrogens in benzene are equivalent. If a hydrogen is substituted by another atom or group, it does not matter which hydrogen is replaced. Only one product is formed. Always remember that in benzene monosubstitution leads to a single product. Disubstitution yields three isomers. All carbon atoms in benzene are sp2-hybridised.
Electrophilic Substitution Reaction of benzene:
Benzene undergoes electrophilic substitution reactions. The benzene ring with its delocalized π electrons is an electron-rich system. It is attacked by electrophiles, giving substitution products. These reactions can be represented as:
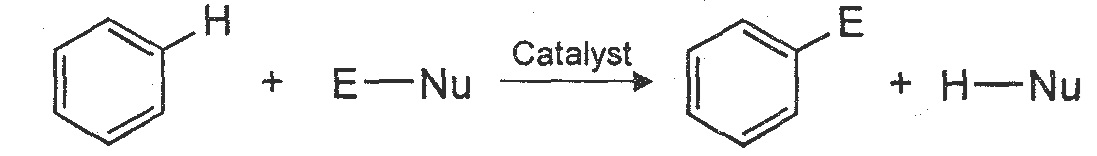
Where E+ is any electrophile and Nu:– is a nucleophile. Such reactions in which hydrogen atom of the aromatic ring is replaced by an electrophile are called electrophilic substitution reactions.
General Mechanism:
All electrophilic substitution reactions follow the same three step mechanism:
Step-1: Formation of an electrophile.
E—Nu + Catalyst ——-> E+ + Nu –Catalyst
Step-2: The electrophile attacks the aromatic ring to form a carbonium ion.
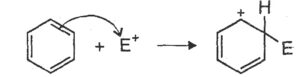
Step-3: Loss of proton gives the substitution product.
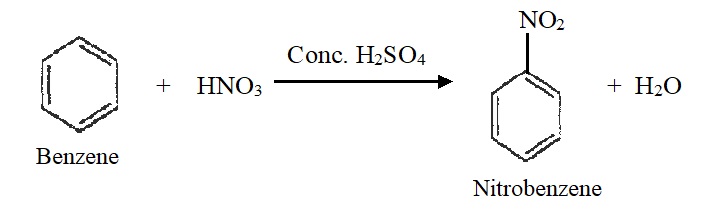
 1) Nitration:
1) Nitration:
Benzene reacts with concentrated nitric acid in presence of concentrated sulphuric acid at 60c to form nitrobenzene.
 2) Sulhponation:
2) Sulhponation:
Benzene reacts with concentrated sulphuric acid at 120c to form benzenesulphonic acid.
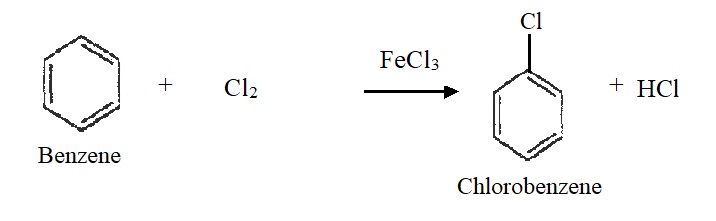
 3) Halogenation:
3) Halogenation:
Benzene reacts with chlorine in presence of FeCl3 or AlCl3 at room temperature to form chlorobenzene.
 4) Friedel-Crafts Alkylation:
4) Friedel-Crafts Alkylation:
Benzene reacts with alkyl halides in the presence of aluminium chloride to form alkylbenzene. For example, 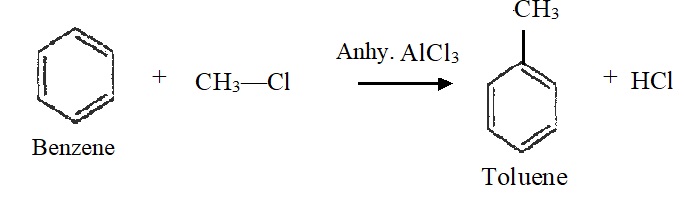 5) Friedel-Crafts Acylation:
5) Friedel-Crafts Acylation:
Benzene reacts with acid chlorides in the presence of aluminium chloride to form aromatic ketones. For example,
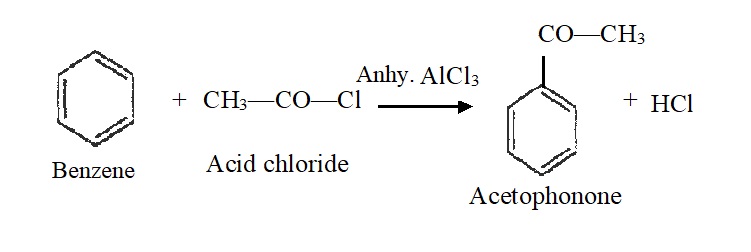 Note:
Note:
- -X, -OH, -OCH3, -NH2, -R, are ortho and para directing group.
- –NO2, -SO3H, -CN, COOH, -CHO, -COR are meta directing group.
- Aromatic compounds are toxic and carcinogenic in nature.