Brownian movement:
The colloidal particles are moving at random in a zig-zag motion. This type of motion is called Brownian movement after the name of its discoverer, Robert Brown, who first observed the zig-zag motion of pollen grains suspended in water.
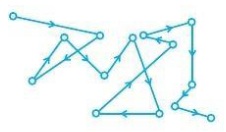
Cause of Brownian movement: The molecules of the dispersion medium are constantly colliding with the particles of the dispersed phase and the impacts of the dispersion medium particles are unequal, thus, causing a zig-zag motion of the dispersed phase particles.