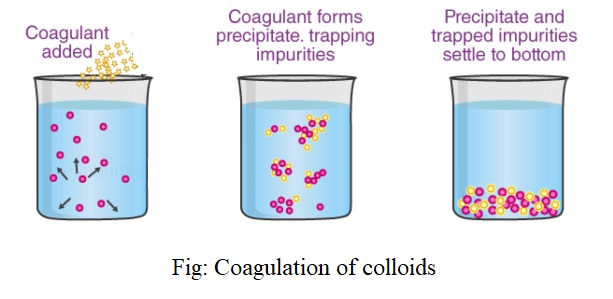
Coagulation: The phenomenon of the precipitation of a colloidal solution by the addition of excess of an electrolyte is called Coagulation or flocculation.
The particles of the dispersed phase i.e. colloids bear some charge. When an electrolyte is added to the colloids, the colloidal particles take up ions carrying opposite charge from the electrolyte. As a result, their charge gets neutralized and this causes the uncharged particles to come closer and get coagulated or precipitated.