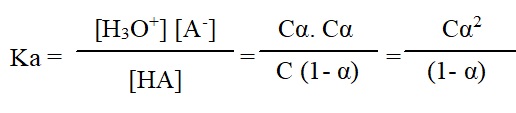Arrhenius Acids and Bases:
An acid is a hydrogen containing compound which gives free hydrogen ions when dissolved in water.
A base is a hydroxyl group containing compound which gives free hydroxyl ions (OH) when dissolved in water.
Thus, according to the Arrhenius concept, hydrogen chloride, acetic acid, and sulphuric acid are acids because all these compounds give free H+ ions in aqueous solution.
HCl (g) + H2O (excess) → H+ (aq) + Cl– (aq)
H2SO4 + H2O (excess) → H+ (aq) + SO4-2 (aq)
CH3COOH + H2O (excess) → H+ (aq) + CH3COO– (aq)
The compounds such as, NaOH and NH4OH are bases, because these compounds give free –OH ions in aqueous solutions.
NaOH + H2O (excess) → Na+(aq) + OH– (aq)
NH4OH + H2O (excess) → NH4+ (aq) + OH– (aq)
Arrhenius concept of acids and base suffers from the limitation of being applicable only to aqueous solutions and do not accounts for the basicity of substances like ammonia which contains no hydroxyl group.
Bronsted-Lowry Acids and Bases:
Any hydrogen containing species (a molecule, a cation or an anion) which is capable of donating one or more protons to any other substance is called an acid.
Any species (molecule, cation or anion) which is capable of accepting one or more protons from an acid is called a base.
Thus, according to the Bronsted-Lowry concept, an acid is a proton-donor and a base is proton acceptor.
The base that is produced when an acid donates its proton is called the conjugate base of an acid. The acid that is produced when a base accepts a proton is called the conjugate acid of a base.
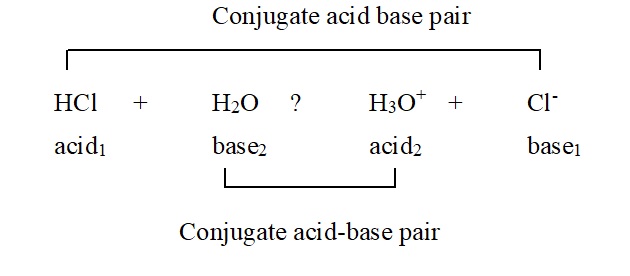
HCl + H2O → H3O+ + Cl–
acid1 base2 acid2 base1
In the reaction, Cl– is the conjugate base of the acid HCl and H2O is the conjugate base of the acid H3O+. Thus, the conjugate acid differs from its conjugate base by one proton.
A pair of an acid and a base which differ from are another by a proton are said to be a conjugate acid-base pair. Thus
 Bronsted acid → conjugate base of the acid + H+
Bronsted acid → conjugate base of the acid + H+
Bronsted Base + H+ → conjugate acid
 Lewis Acids and Bases:
Lewis Acids and Bases:
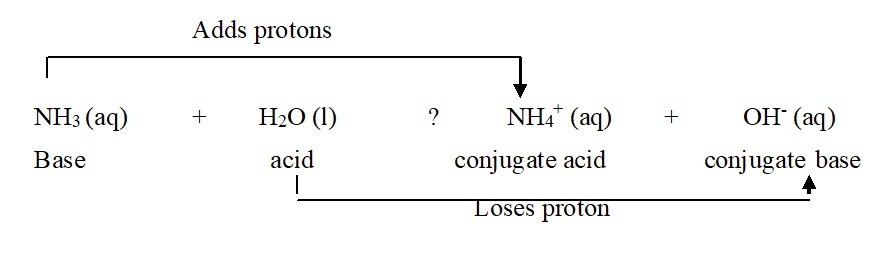
Lewis acid is a substance (molecules or ion) which can accept a pair of electrons while a base is a substance (molecules or ion) which can donate a pair of electrons. e.g. BF3 is a Lewis acid accepts a pair of electrons while: NH3 is a base donates a pair of electrons.
Ionisation of Acids and Bases:
The strength of acid or base is determined with the help of extent of ionization in aqueous solution. An acid of the type HA (e.g. HC1, CH3COOH) undergoes ionization when dissolved in water in accordance with the reaction,
HA + H2O ═ H3O+ + A–
If n moles of the acid are dissolved in V units of volume (say litre, L) and α is the degree of ionization at that concentration, then the equilibrium amounts of various species in the solution are
Number of moles of HA = n (1- α)
Number of moles of H3O+ = n α
Number of moles of A– = n α
The corresponding concentrations in moles per litre are
[HA] = n (1- α)/ V mol L-1 = c (1- α) mol L-1
[H3O+] = n α/ V mol L-1 = cα mol L-1
[A–] = n α/ V mol L-1 = cα mol L-1
Where ‘C’ is the molar concentration of the acid. Then, the ionization constant for the acid HA is given by,