Electrochemistry (electrochemical cell, Electrolytic cell, Salt Bridge, Representation of galvanic cell):
Electrical energy plays an important role in many chemical reactions. The branch of science which deals with the relationship between electrical energy and chemical energy and interconversion of one form into another is called electrochemistry. These may include chemical reactions which occur due to the passage of electrical energy as well as the generation of electrical energy due to chemical equation. The basis of these types of processes is redox reactions.
The chemical changes which involve the flow of electric current are called electrochemical changes. These are two types:
- Electrolytic cell
- Galvanic cell or electrochemical cell
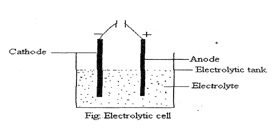
Electrolytic cell: Electrolytic cell is a device in which the electrical energy is converted into chemical energy resulting in a chemical reaction. The electrode connected to the negative terminal of the battery is the Cathode. On the other hand, the electrode connected to the positive terminal of battery is called the Anode. The passage of electricity through the electrolyte leads to its decomposition and this process is called electrolysis. For example, when electric current is passed though molten/ aq. solution of sodium chloride, sodium is deposited at cathode and chlorine is liberated at anode.
NaCl ———> Na+ + C1–
At cathode, Na+ +e ———-> Na (Reduction)
At anode, Cl– – e ————> Cl (Oxidation)
Galvanic cell or Electrochemical cell: Galvanic cells constitute the electrochemical reactions in which chemical energy is converted to electrical energy. In this cell, spontaneous redox reaction is used to generate an electric current. This is reverse of electrolysis.
The devices in which chemical energy released during a chemical reaction is converted into electrical energy are called Galvanic cells or Electrochemical cells. In order to understand, this phenomenon, let us consider Zn—CuSO4 reaction as the basis of the cell.
In its simple form, a zinc rod is dipped in ZnSO4 solution and a copper rod is dipped in CuSO4 solution taken in a separate beakers. The two metal electrodes connected by a wire through a voltmeter. The two solutions is joined by an inverted U-tube filled with saturated semi-solid paste of some electrolytes such as KC1, KNO3, NH4CI mixed with gelatin or agar-agar, called salt bridge. The deflection in voltmeter indicates that there is a potential difference between the two electrodes. It has been found that the conventional current flows through the enter circuit from Cu to Zn. It implies that the electron flows from zinc to copper.
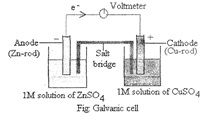
Let us consider the working of cell.
- Zn undergoes oxidation to form Zn+2 Zn (s) ——->Zn+2+ 2e (Oxidation)
- The electrons liberated during oxidation are pushed through the connecting wires to copper electrode.
- Cu+2 ions move towards copper electrode, pick up the electrons and get reduced to Cu-atom which is deposited at the copper electrode. Cu+2 + 2e ——-> Cu (s) (Reduction)
The electrode at which oxidation occurs, called Anode and at which reduction occurs, called Cathode. In the above cell, the Zn-rod is anode and that of Cu-rod is cathode. Due to oxidation process occurring at the anode, it becomes a source of electrons and acquires a negative charge, Similarly, due to reduction occurring at the cathode it acquires positive charge and becomes a receiver of electrons.
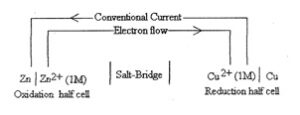
Representation of galvanic Cell: Galvanic cell is a combination of two half-cell namely oxidation half-cell and reduction half-cell. If M represents the element and Mn+ represents its Cation in solution, then
Oxidation half-cell is represented as- M | Mn+(c)
Reduction half-cell is represented as— M n+(c)| M
Where c refers to the molar concentration of the ions in solution. Conventionally, a cell is represented by writing the cathode on the right hand side and anode on the left hand side. The two vertical tines are put between the two half cells which indicates salt bridge. e.g. Zn—CuSO4 cell is represented as —
Salt Bridge: Salt Bridge is U-tube containing a semi-solid paste of some inert electrolyte like KC1, KNO3, NH4Cl mixed with gelatin or agar-agar. An electrolyte is one which— (i) Do not react chemically with the solution in either of the compartment and (ii) Do not interfere with the net cell reaction, a salt bridge serves two very important functions:
- It allows the flow of current by completing the circuit
- It maintains the electrical neutrality.