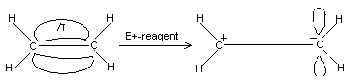
Electromeric effect:
“The electromeric effect (E-effect) refers to the polarity produced in a multiple bonded compound as it is approached by a reagent”.
When a double or a triple bond is exposed to an attack by an electrophile (E+), a reagent, the two π-electrons which form the π-bond are completely transferred to one atom or the other. The electromeric effect is represented as:
![]()
The curved arrow shows the displacement of electron pair. The atom A has lost its share in the electron pair and B has gained this share. As a result, A acquires a positive charge and B a negative charge. The electromeric effect is a temporary effect. It takes place only in the presence of reagent.