Exceptional Stability of half-filled and full-filled sub-shell:
It may be noted that the configuration of chromium (Z=24) and cupper (Z=29) do not follow the general rule. For these elements, the expected and actual electronic configurations in ground state are as follows —
Expected configuration of Cr and Cu:
Cr (Z=24) = 1s22s22p63s23p63d44s2
Cu (Z=29) = 1s22s22p63s23p63d94s2
Actual electronic configuration of Cr and Cu :
Cr (Z=24) = 1s22s22p63s23p63d54s1
Cu (Z=29) = 1s22s22p63s23p63d104s1
The actual electronic configurations of these elements have been found to be extra stable than expected electronic configuration because of the following two reasons —
1) Symmetry of orbital:
The configuration in which all the degenerate orbital of the same sub-shell are half filled or completely filled have symmetrical distribution of electrons. Such electronic configurations are more stable than those in which the distribution is not symmetrical. As symmetry leads to stability, such electronic configuration, wherever they are possible, are preferred.
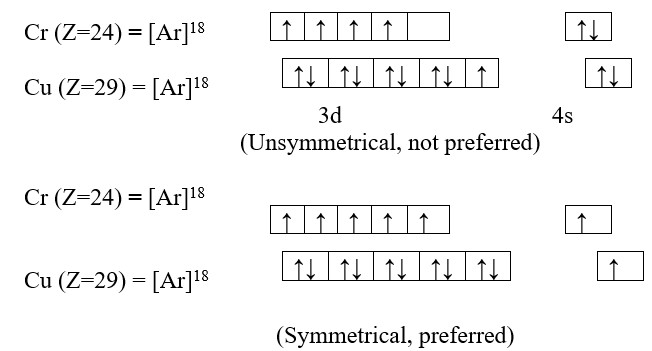 2) Exchange Energy:
2) Exchange Energy:
In an atom, electrons present in various orbital of the same sub-shell tend to exchange their position mutually and during this process small amount of energy is lost. It is called exchange energy. Larger the exchange energy, greater is the stability. Maximum exchange energy of electron is possible in half filled and completely filled sub-shells so that maximum amount of exchange energy is released in this configuration. This allows for greater stability in such symmetrical configuration than the unsymmetrical configuration. Therefore, electronic configuration 3d54s1and 3d104s1 are preferred over respective 3d44s2and 3d94s2.
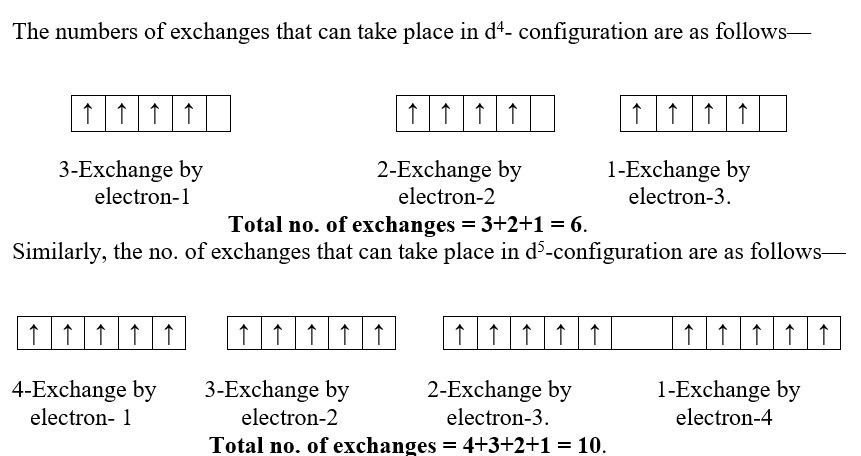
We find that more exchanges are possible in d5-configuration than d4-configuration. Similar arrangement can be extended to explain the extra stability of 3d104s1 configuration than 3d94s2.