Q. 1) Explain the term Inductive. Which electron displacement effect explains the following correct orders of acidity of the carboxylic acids?
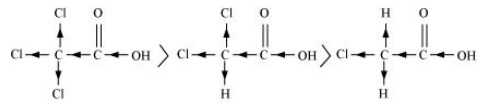
(a) Cl3CCOOH > Cl2CHCOOH > ClCH2COOH
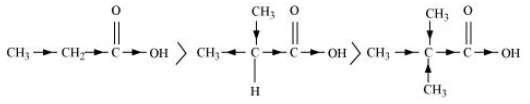
(b) CH3CH2COOH > (CH3)2CHCOOH > (CH3)3C.COOH
Ans:
Inductive effect:
The permanent displacement of sigma electrons along a saturated chain, whenever an electron withdrawing or electron donating group is present, is called inductive effect. Inductive effect could be + I effect or – I effect. When an atom or group attracts electrons towards itself more strongly than hydrogen, it is said to possess – I effect. For example,
F —← CH2 —← CH2 —← CH2 —←CH3
When an atom or group attracts electrons towards itself less strongly than hydrogen, it is said to possess + I effect. For example,
CH3 —→ CH2 →→— Cl
(a) Cl3CCOOH > Cl2CHCOOH > ClCH2COOH
The order of acidity can be explained on the basis of Inductive effect (- I effect). As the number of chlorine atoms increases, the – I effect increases. With the increase in – I effect, the acid strength also increases accordingly.
(b) CH3CH2COOH > (CH3)2 CHCOOH > (CH3)3 C.COOH
The order of acidity can be explained on the basis of inductive effect (+ I effect). As the number of alkyl groups increases, the + I effect also increases. With the increase in + I effect, the acid strength also increases accordingly.