Isomerism in coordination compounds: The two or more compounds having the same molecular formula but different properties are called Isomers and the phenomenon is called Isomerism. Isomers are classified into two major type namely structural isomerism and stereoisomerism.
Structural Isomers: The isomers which have same molecular formula but different structural arrangement of atoms or groups of atoms around the central metal ion are called structural isomers. The structural isomers are further classified as Ionisation Isomerism, Hydrate Isomerism, Coordination isomerism and Linkage Isomerism.
Ionisation Isomerism: The compounds which have same molecular formula but give different ions in solution are called ionization isomers. e.g. there are two isomers of the compound of the formula [Co (NH3)5 Br]SO4. The structures of the two compounds and their mode of ionization are:
[Co (NH3)5 Br]SO4 —————-> [Co (NH3)5 Br]2+ + SO4-2
Pentaamminebromocobalt (III) sulphate Gives test of SO42- ions.
[Co (NH3)5SO4] Br ————–> [Co (NH3)5SO4]+ + Br –
Pentaamminesulphatocobalt (III) bromide Gives test of Br– ions.
Hydrate Isomerism: The compounds which have same molecular formula but differ in the number of water molecules present as ligands or as molecules of hydration are called hydrate isomers. e.g. there are three isomers having the molecular formula CrCl3.6H2O. These are –
[Cr(H2O)6]Cl3-Violet, [Cr(H2O)5Cl]Cl2.H2O- Blue and [Cr(H2O)4Cl2]Cl.2H2O- Green.
Coordination Isomerism: Coordination isomerism occurs in compounds containing both cationic and anionic complexes and the isomers differ in the distribution of ligands in the coordination sphere of cationic and anionic parts. e.g.
[Co(NH3)6][Cr(CN)6] and [Cr(NH3)6][Co(CN)6]
Linkage Isomerism: The compounds which have the same molecular formula but differ in the mode of attachment of a ligand to the metal atom or ion are called linkage isomers. e.g. in NO2– ion, the nitrogen atom (nitro) as well as the oxygen atom (nitrito) can donate their lone pairs.
[Co(NH3)5(NO2)]Cl2 Pentaamminenitrocobalt (III) chloride
Yellow brown
[Co(NH3)5(ONO)]Cl2 Pentaamminenitritocobalt (III) chloride
Red
Stereoisomers: Stereoisomers are those isomers which have the same position of atoms or groups but they differ in the spatial arrangements around the central atom. Stereoisomerisms are two types viz., geometrical isomerism and optical isomerism.
Geometrical Isomerism: Geometrical isomerism is due to ligands occupying different positions around the central ion. The ligands occupy positions either adjacent to one another or opposite to one another. These are referred to as cis-form (ligands occupy adjacent positions) and trans-form (ligands occupy opposite positions). This type of isomerism is, therefore, also called cis-trans isomerism.
Geometrical isomerism in complexes of coordination number 4: The complex having coordination number 4 adopt tetrahedral or square planar geometry. The geometrical isomerism is not possible in tetrahedral complexes. This is because in tetrahedral geometry all the positions are adjacent to one another in these complexes. However, square planar complexes show geometrical isomerism. For examples,
[Pt(NH3)2Cl2] exists in cis and trans forms as: 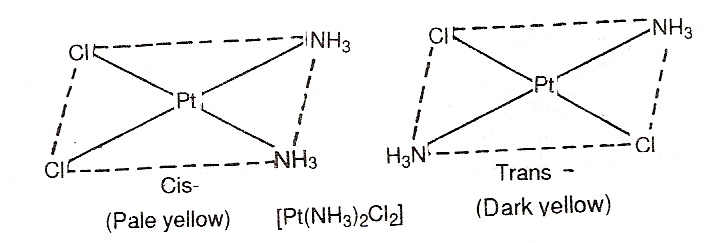 [Pt(py)2(NH3)Cl] exists in cis and trans form as:
[Pt(py)2(NH3)Cl] exists in cis and trans form as:
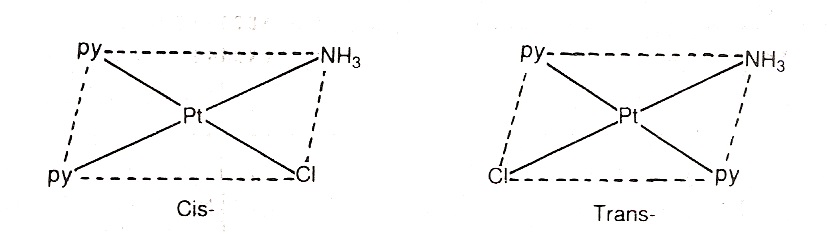 Geometrical isomerism in complexes of coordination number 6: The complexes having coordination number 6 adopt octahedral geometry. For examples,
Geometrical isomerism in complexes of coordination number 6: The complexes having coordination number 6 adopt octahedral geometry. For examples,
An octahedral complex [Co(NH3)4Cl2]+ can exist as cis and trans-isomers-
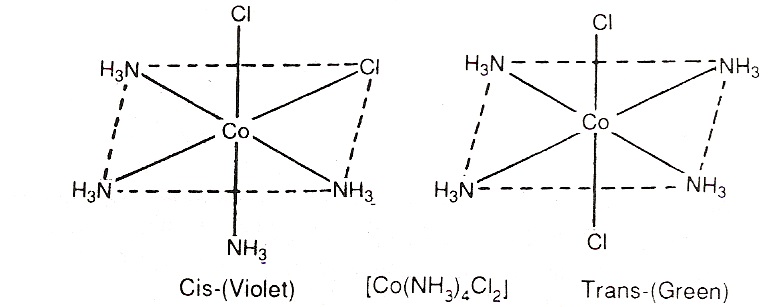
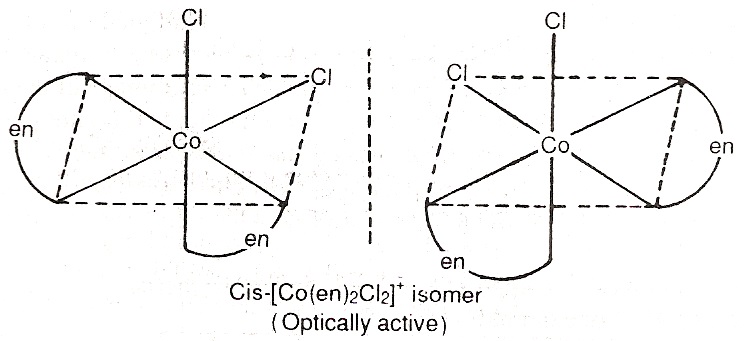
Optical Isomerism: There are some substances which can rotate the plane of polarized light. These are called optically active substances. The isomer which rotates the plane of polarized light equally but in opposite direction are called optical isomers. The isomer which rotates the plane of polarized light to the right is called dextro rotatory (d) and the isomer which rotates the plane of polarized light to the left is called laevo rotatory (l).
For example, complex [Co(en)2CL2]+ forms geometrical isomers (cis and trans form). It is noted that the trans form does not show optical isomerism i.e. it can not be resolved into optical isomers. The reason is that the molecule has a plane of symmetry. On the other hand, the cis-isomer is unsymmetrical and can be resolved into optical isomers.
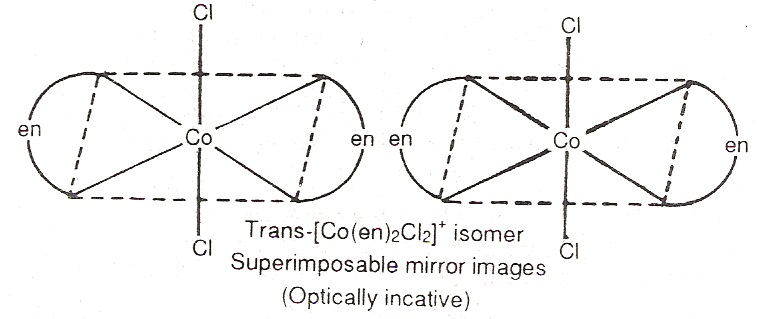
Bonding in coordination compounds: To explain some of the properties of coordination compounds such as colour, geometry and magnetic properties, three main theories namely valence bond theory, crystal field theory and molecular orbital theory have been proposed.
VBT (Valence Bond Theory) for coordination compounds: The main assumptions of this theory are as follows—
1) The central metal ion in the complex makes available a number of empty orbitals for the formation of coordinate bonds with suitable ligands. The number of empty orbitals made available for this purpose is equal to the number of the central metal ion. e.g. if coordination number is six, 6 empty orbitals are made available and if coordination number is 4, four empty orbitals are made available in the central metal ion.
2) The appropriate atomic orbitals (s, p and d) of the metal hybridise to give a set of equivalent orbitals of definite geometry such as square planar, tetrahedral, and octahedral and so on.
Coordination number | Hybridisation | Geometry | |
4 | sp3 | Tetrahedral | |
4 | dsp2 | Square planar | |
| 6 | sp3d2 or d2sp3 | octahedral | |
3) The d-orbital involved in the hybridization may be either inner d-orbitals i.e. (n-1) d or outer d-orbitals i.e. nd. e.g. in the case of octahedral hybridization, the orbitals may be two 3d, one 4s and three 4p (d2sp3) or one 4s, three 4p and two 3d (sp3d2 hybridisation).
4) Each ligand has at least one orbital (of donor atom) containing a lone pair of electrons.
5) The empty hybrid orbital of metal ion overlap with the filled orbitals of the ligands to form metal-ligand coordinate covalent bonds.
Application of valence bond theory: Let us consider some examples to illustrate valence bond theory—
[Cr(NH3)6]3+: In this complex, the chromium ion is in +3 oxidation state and has an electronic configuration of 3d3 , as shown below.
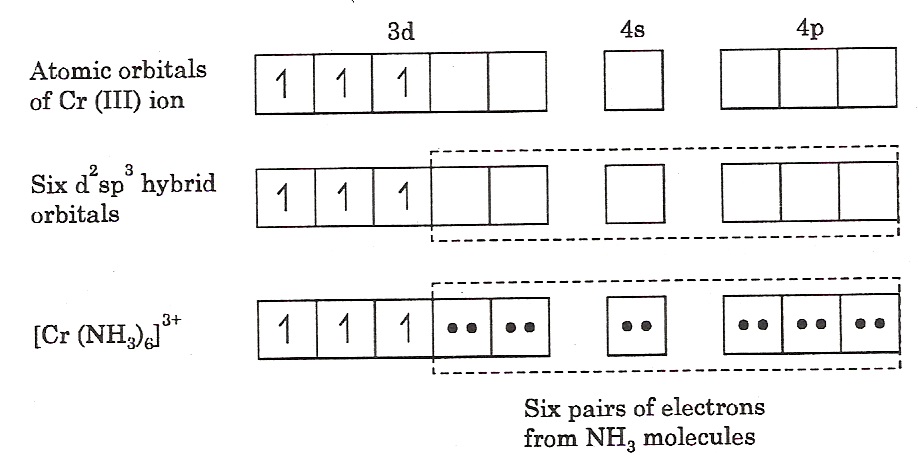
The two 3d, one 4s and three 4p orbitals then hybridise to yield six d2sp3 hybride orbitals pointing towards the six ends of an octahedron. The six ammonia molecules then donate a pair of electrons to each of the vacant orbitals. Consequently the structure of the complex is octahedral. The presence of three unpaired electrons in the remaining orbitals of Cr(III) makes the complex paramagnetic.
Examples of complexes of coordination number 4: In this case, the geometry will be tetrahedral or square planar depending upon whether sp3 or dsp2-hybridisation is involved.
[Ni(CN)4]2-, tetracyanonickelate (II) ion: It is a square planar complex in which nickel is in +2 oxidation state and has the electronic configuration of 3d8. In this case, the two unpaired electrons of the 3d orbitals are forced to pair in the presence of strong ligand, CN– ion. Since four ligands are to be accommodated, therefore, nickel (II) ion undergoes dsp2 hybridisation forming four equivalent dsp2 hybrid orbitals. These accommodate the four pair of electrons from the ligands.
In the formation of the complex since the inner d-orbital is used in hybridization, [Ni(CN)4]2- is called an inner orbital or spin paired or low spin or hyperligated complex. The hybridization of the above complex is as follows: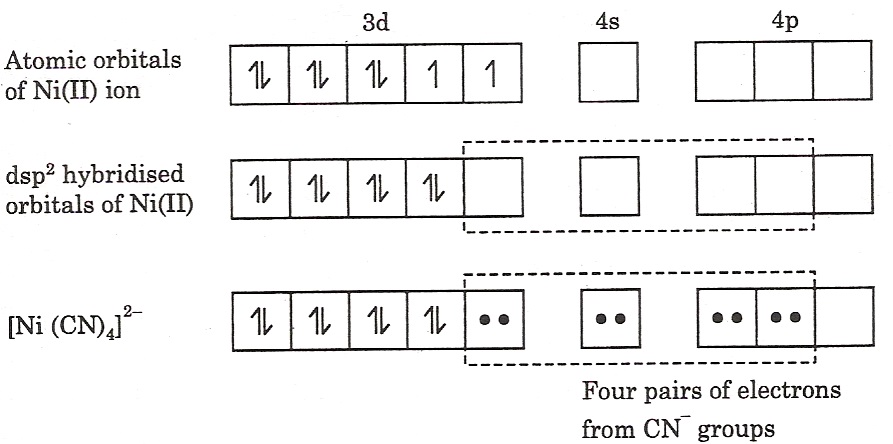 As there are no unpaired electrons, the complex is diamagnetic and because of dsp2-hybridisation of nickel it has square planar geometry.
As there are no unpaired electrons, the complex is diamagnetic and because of dsp2-hybridisation of nickel it has square planar geometry.
Drawbacks of valence bond theory:
1) It is a quantitative approach describing bonding in co-ordination compounds.
2) The theory fails to explain the optical absorption spectra and magnetic properties of coordination compounds.
3) This theory does not give exact explanation regarding structures of 4-co-ordinate complexes i.e. whether these are tetrahedral or square planar.
4) It does not distinguish between strong and weak ligands.
5) This theory does not give any idea of kinetic stability of coordination compounds.
Colour of complex ions:
The absorption of electro magnetic radiations by an ionic species in solution requires that electrons within the ion undergo a transition from one energy level to another. To cause this transition a suitable wavelength component is absorbed and the transmitted light corresponds to a particular colour.
We know that crystal field splitting of the d-energy levels produces the energy difference, Δ. This value of energy difference is usually small. Thus, promotion of electrons from lower to higher d-level results from the absorption of an appropriate component (or wavelength) of white light. In simple words the intra d-d transitions occurs and account for the colour of complex ions and their compounds.
The colour of a particular compound depends upon the magnitude of Δ, the crystal field splitting which is further found to be dependent upon the nature of ligand (or ligand field). e.g.
Octahedral complexes of chromium (III) are all d3 systems, yet the colour of the complexes is different. It is because different ligands present around the central metal ion produce different ligand fields and hence different crystal field splitting (Δ). The magnitude of splitting observed in each case can be explained on the basis of spectrochemical series.
Isomer Colour
[Cr(H2O)6]Cl3 violet
[Cr(H2O)4 Cl2]Cl green
Magnetic properties: The transition metal complexes are known to be paramagnetic in character. CFT helps us to understand the magnetic properties in terms of magnetic susceptibility measurements. The magnetic properties of a substance depend upon the oxidation state, electronic configuration, coordination number of central metal and the nature of ligand field. An unpaired electron because of its spin is equivalent to an electric current flowing in a circular conductor. Hence, it behaves as a magnet.
The magnetic moment of a substance depends upon the number of electrons i.e. greater is the number of unpaired electrons more is the magnetic moment. The magnetic moment of a substance containing ‘n’ unpaired electrons is given by the expression as—
Magnetic moment, μ= √n (n+2) Bohr magnetons
From the knowledge of number of unpaired electrons and the value of magnetic moment (μ) it is possible to find
- Valence state of the metal ion in a given complex.
- Natures of bonding in the complex i.e. spin free or spin paired type.
Importance of coordination compounds
The ability of metal ions to form complexes with a variety of molecular species with different physico-chemical properties has been used in many ways. In the recent years, the complexes and the complex formation methods have been finding extensive uses. Some of these are as follows—
1) In qualitative analysis: The complex formation method is used in qualitative analysis of group-I for the separation of silver ion from the precipitate of AgCl,Hg2Cl2 and PbCl2. We add aqueous ammonia solution to the precipitate when AgCl dissolves due to the formation of the complex [Ag(NH3)2]+ ion.
AgCl + 2NH3 ![]() [Ag(NH3)2]Cl
[Ag(NH3)2]Cl
Soluble
Hg2Cl2 and PbCl2 do not form complexes and therefore, do not dissolve.
Cd2+ ions can be tested in the presence of Cu2+ ion by forming their complexes with KCN. Copper forms [Cu(CN)4]3- which ionizes less than [Cd(CN)4]2-. On passing H2S only cadmium ions will be precipitated as CdS while Cu2+ ions will not ppt. This application is also called masking of ions.
2) In the extraction of metals: Complex formation techniques are also used for the extraction of metals such as gold and silver. For examples, silver is extracted from its ores by the cyanide process. In this process, silver passes into solution with the formation of complex Na[Ag(CN)2].
3) In biological system: Coordination compounds play many important roles in animals and plants. They are essential in storage and transport of oxygen, as electron transfer agents, as catalysts and in photosynthesis.
- In chlorophyll the central ion is Mg2+ whereas
- In haemoglobin it is Fe2+ ion