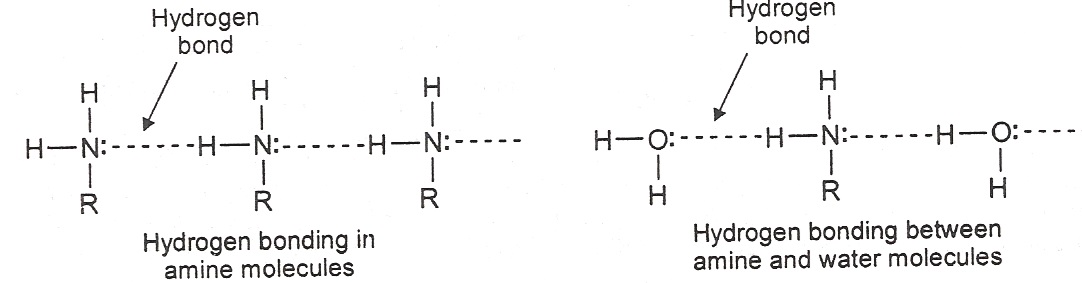
Physical Properties: Lower amines are gases or low boiling liquids and possess characteristic ammonia like smell. Amines have higher boiling points than non-polar compounds of similar molecular weights. This is because amines are polar compounds and form hydrogen bonds. e.g. ethylamine, CH3CH2NH2, boils at 17ºc and propane C3H8, at -42ºc. Amines are soluble in water because of h-bonding with water. Amines are also soluble in benzene and ether.
Basic Character of amines: Amines are basic in nature. This is because they posses an unshared pair (lone pair) of electrons on nitrogen. This lone pair of electrons is available for the formation of a new bond with a proton or Lewis acids. Thus amine reacts with acids to form salts
R—NH2 + H—Cl ———> R—N+H3 Cl–
Amine Alkylamonium chloride
Aliphatic amines are stronger bases than ammonia. This is because the alkyl groups are electron-releasing. They increase the electron density around the nitrogen, thereby increasing the availability of the lone pair of electrons. The greater the number of electron releasing alkyl groups, greater is the availability of nitrogen’s lone pair and stronger the base.
The greater the number of electron releasing alkyl groups, greater is the availability of nitrogen’s lone pair and stronger the base.
![]() Notice that the dimethylamine is a stronger base than methylamine. However, trimethylamine is a weaker base than both dimethylamine and methylamine. Although in trimethylamine the electron-density is further increased, the steric crowding of three methyl groups makes the approach and bonding by a proton relatively difficult. The electrons are there but the path is blocked.
Notice that the dimethylamine is a stronger base than methylamine. However, trimethylamine is a weaker base than both dimethylamine and methylamine. Although in trimethylamine the electron-density is further increased, the steric crowding of three methyl groups makes the approach and bonding by a proton relatively difficult. The electrons are there but the path is blocked.
Comparison of basic strength of aniline (aromatic) and ethylamine (aliphatic): Both aliphatic and aromatic amines are basic in nature due to the presence of lone pair of electron on nitrogen atom. But aniline is less basic than ethylamine. The less basic character of aniline can be explained on the basis of aromatic ring presence in aniline. Aniline can have the following resonating structures:
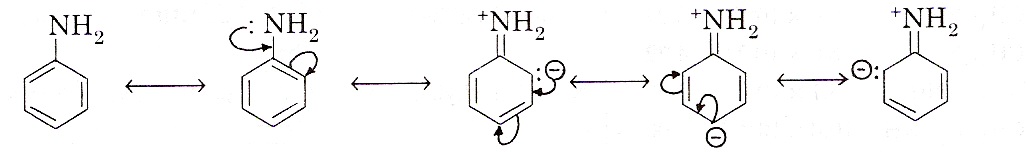
It is clear from the above resonating structures these structures acquire some positive charge on nitrogen. As a result, the pair of electrons becomes less available for protonation.
Hence, aniline is less basic than ethylamine in which there is no such resonance.
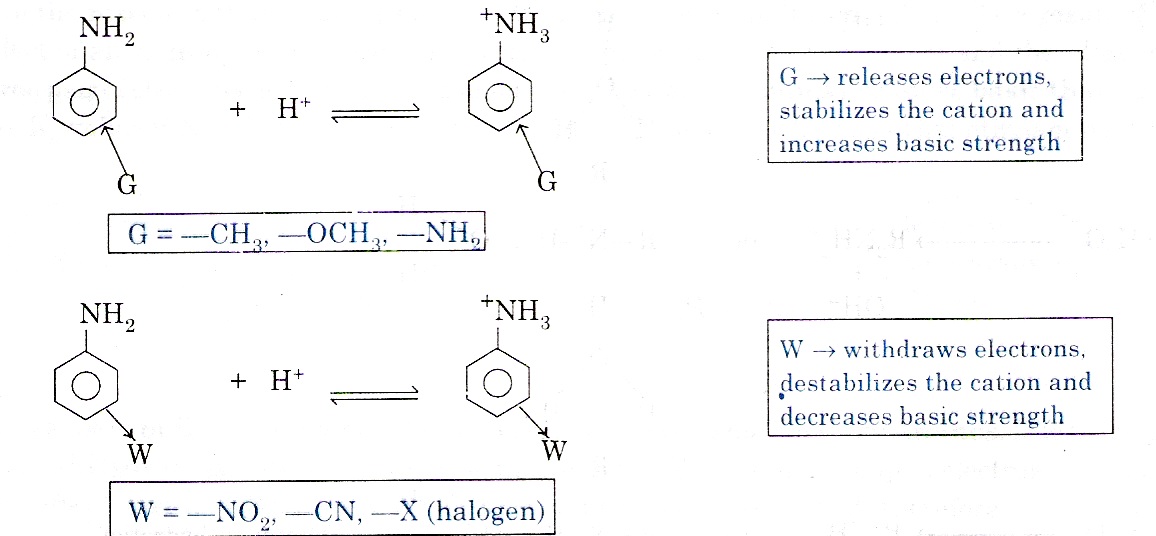
Effect of substituents on the basic character of aromatic amines: Electron releasing group like –OCH3, ─CH3 increase the basic character, while electron withdrawing groups like –X (halogen), ─NO2, ─CN decreases the basic character.
Chemical reaction of amines:
The main reactions of amines are due to the presence of the lone pair of electrons on nitrogen. This lone pair of electrons is available for donation to electron-seeking reagents. Amines are nucleophilic reagents.
(1) Reaction with nitrous acid: Nitrous acid (HONO) is an unstable substance and is therefore prepared in situ by the reaction of sodium nitrite with dilute HCl at 0ºc.
NaNO3 + HCl ————–> HONO + NaCl
- Primary amine reacts with nitrous acid to form alcohols and nitrogen gas.
R—NH2 + HONO————–> R—OH + N2 + H2O
1º-amine alcohol
C2H5—NH2 + HONO ————> C2H5—OH + N2 + H2O
- Secondary amines react with nitrous acid to form N-nitrosoamines which are water insoluble yield yellow oils.
R2—NH + HONO—————-> R2—N—N=O + H2O
2º-amines
(C2H5)2—NH + HONO ———-> (C2H5)2—N—N=O + H2O
N-Nitrosodiethylamine (yellow oil)
(c) Tertiary amines react with nitrous acid to form trialkylammonium nitrate salts which are soluble in water.
R3—N + HONO —————-> R3-N+HNO2–
(C2H5) 3 –N + HONO ————> (C2H5) 3 – N+HNO2.
3º-amines Trimethylammonium nitrite (soluble)
This reaction is used to distinguish between primary, secondary and tertiary amines. The test is known as the Nitrous acid test.
(2) Carbylamine reaction: Primary amine reacts with alcoholic solution of KOH to form isocyanides or carbylamine. Secondary and tertiary amines do not give this reaction.
R—NH2 + CHCl3 + 3KOH ————-> R—N+≡C– + 3KCl + 3 H2O
1º-amine isocyanide
This reaction is used to distinguish primary amines from secondary and tertiary amines.
Some important reaction of aniline:
(i) Halogenation of aniline: Aniline reacts with bromine water readily to give a white precipitate of 2, 4, 6-tribromoaniline.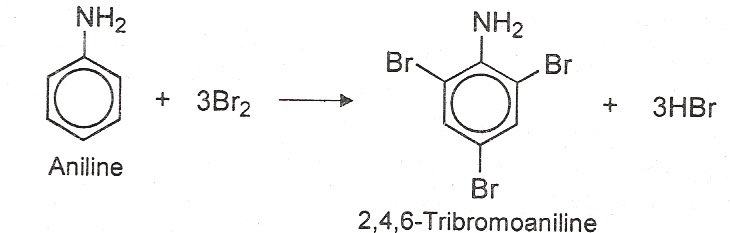 (ii) Reaction with nitrous acid: Aniline reacts with nitrous acid in presence of HCl at 0ºc to give benzenediazonium chloride.
(ii) Reaction with nitrous acid: Aniline reacts with nitrous acid in presence of HCl at 0ºc to give benzenediazonium chloride.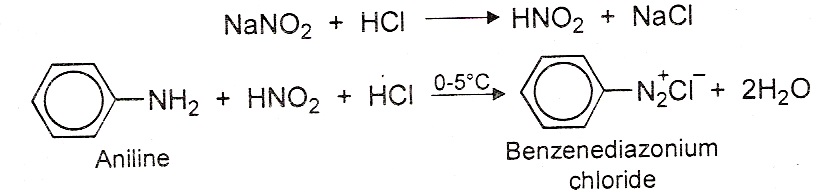
(iii)Coupling with diazonium salts: Aniline reacts with benzene diazonium chloride to give p-aminoazobenzene.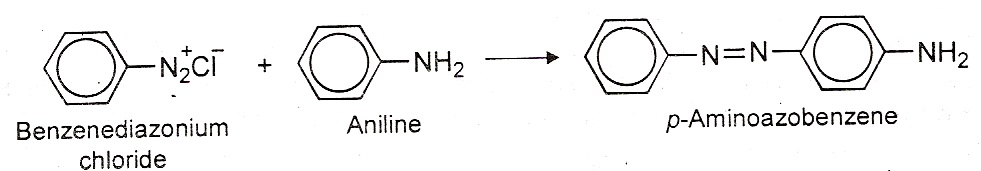 (iv) Nitration: Aromatic amines cannot be nitrated directly because they are readily oxidized. This is because; HNO3 is a strong oxidizing agent and results in partial oxidation of the ring to form a black mass. Therefore, nitration is carried out by protecting the –NH2 group by acetylation. The acetylation deactivates the ring and controls the reaction to monosubstitution stage.
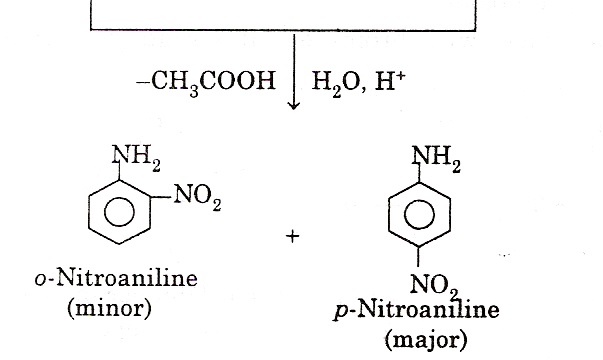
(iv) Nitration: Aromatic amines cannot be nitrated directly because they are readily oxidized. This is because; HNO3 is a strong oxidizing agent and results in partial oxidation of the ring to form a black mass. Therefore, nitration is carried out by protecting the –NH2 group by acetylation. The acetylation deactivates the ring and controls the reaction to monosubstitution stage.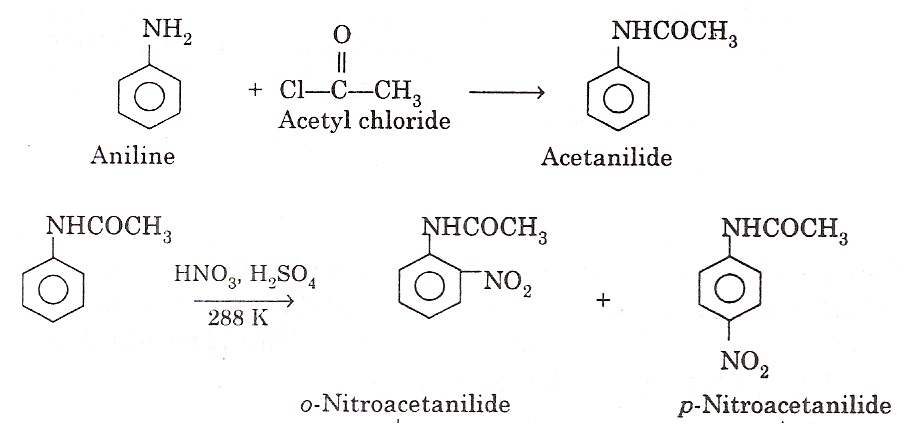
 Separation of mixture of primary, secondary and tertiary amines: The mixture of primary, secondary, tertiary amines and quaternary salt is distilled with KOH, when the amines distil over leaving behind the non-volatile quaternary salt. The mixture of amines obtained as distillate is separated by any of the following methods.
Separation of mixture of primary, secondary and tertiary amines: The mixture of primary, secondary, tertiary amines and quaternary salt is distilled with KOH, when the amines distil over leaving behind the non-volatile quaternary salt. The mixture of amines obtained as distillate is separated by any of the following methods.
- Fractional distillation: The mixture of amines may be separated by fractional distillation because their boiling points are different. This method has been proved to be most satisfactory and efficient and has been extensively used in industry.
- Hofmann’s method: The mixture of primary, secondary and tertiary amines is treated with diethyl oxalate, when primary amine forms solid oxamide, secondary amine forms a liquid oxamic ester while tertiary amine remains unreacted.
(COOC2H5)2 + 2H—NHR ———–> (CONHR)2 + 2C2H5OH
Diethyl oxalate Primary amine N, N-Dialkyl oxalate (Solid)
(COOC2H5)2 + H—NR2 —————> (CONR2)COOC2H5 + 2C2H5OH Diethyl oxalate Secondary amine N, N-Dialkyl oxalate (Liquid)
(COOC2H5)2 + NR3 ————–> No reaction
Diethyl oxalate Tertiary amine
The mixture containing oxamide, oxamic acid and tertiary amine is distilled, when tertiary amine being most volatile, distils over and collected. Now, the residual mixture in the distillation flask contains oxamide and oxamic ester. The solid oxamide is separated from oxamic ester by simple filtration and both are then separately treated with strong alkali regenerating corresponding amines. These are collected and purified by distillation.