Rutherford’s Atomic Model:
Rutherford proposed a model of the atom which is named after him. This is also called the Nuclear Atom. According to it—
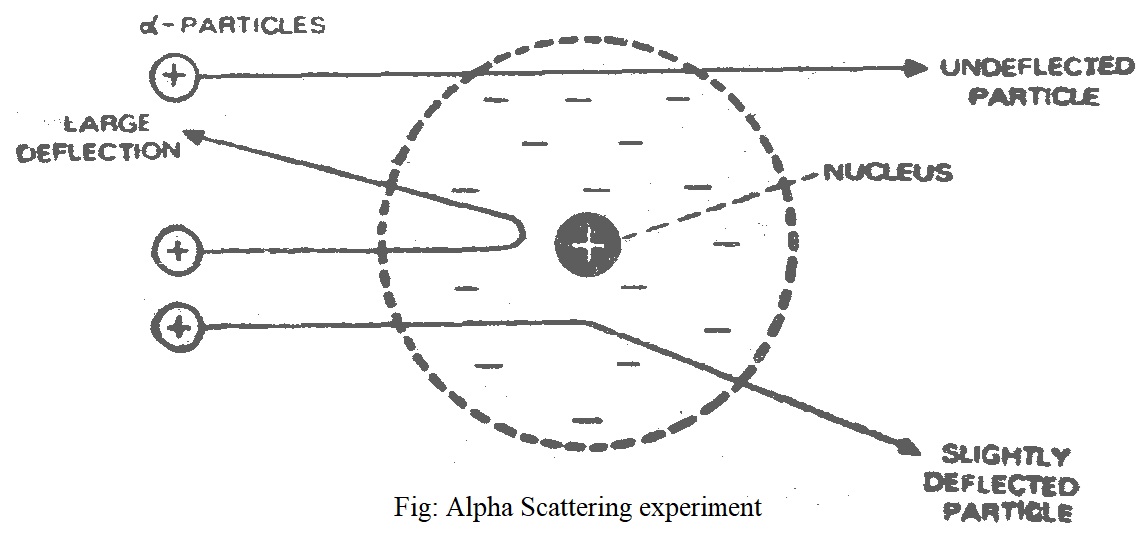
1) Atom has a tiny dense central core or the nucleus which contains practically the entire mass of the atom, leaving the rest of the atom almost empty. It was this empty space around the nucleus which allowed the α-particles to pass through undeflected.
2) The entire positive charge of the atom is located on the nucleus, while electrons were distributed in vacant space around it. It was due to the presence of the positive charge on the nucleus that a- particles (He+2) were repelled by it and scattered in all direction.
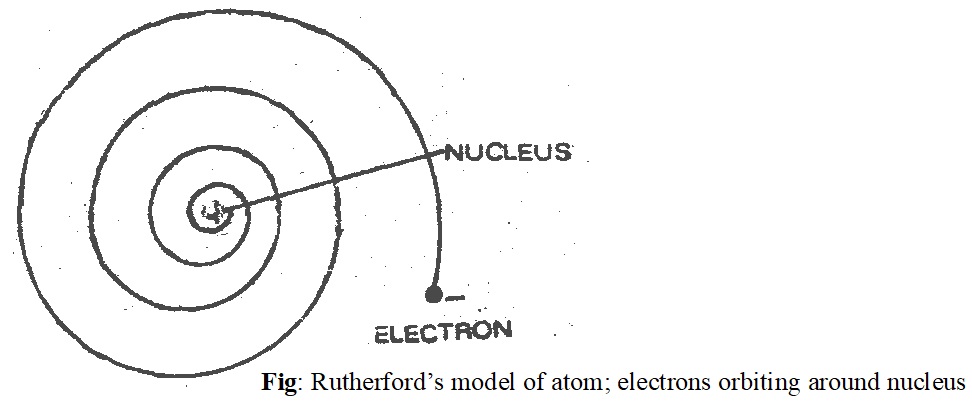
3) The electrons were moving in orbits or closed circular paths around the nucleus like planets around the sun.
Limitation of Rutherford’s Atomic Model:
The assumption that electrons were orbiting about the nucleus was unfortunate. According to classical electromagnetic theory, if a charged particle accelerates around an oppositely charge particle, the former will radiate energy. If an electron radiates energy, its speed will decrease and it will go into spiral motion, finally falling into the nucleus. This does not happen as then the atom would be unstable which it is not. This was the weakness of Rutherford’s atomic model.