Standard Electrode Potential:
Since a half cell in an electrochemical cell can work only in combination with the other half cell and does not work independently; it is not possible to determine the absolute electrode potential of an electrode. We can, therefore, find only the relative electrode potential.
This difficulty can be solved by selecting one of the electrodes as a reference electrode and arbitrarily fixing the potential of this electrode as zero. For this purpose, reversible hydrogen electrode has been universally accepted as a reference electrode. It is called standard hydrogen electrode (S.H.E.) or normal hydrogen electrode (NHE).
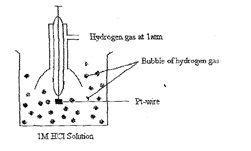
Standard hydrogen electrode:
It consists of platinum wire sealed in a glass tube and has a platinum foil attached to it. The foil is coated with finely divided platinum arid acts as platinum electrode. It is dipped into an acid solution containing H-ions in I M concentration (1M HCI). Pure hydrogen gas at I atmosphere pressure is constantly bubbled into solution at constant temperature of 298 K. The surface of the foil acts as a site for the reaction.
The following reactions occur in this half-cell depending upon whether it acts as anode or as cathode.
if S.H.E acts as Anode, H2(g) ———> 2H+ +2e
if S.H.E acts as cathode, 2H+ + 2e ———> H2 (g)